FLCCC Alliance implores CDC, FDA to review research on Ivermectin as COVID-19 treatment
01/03/2021 / By Divina Ramirez

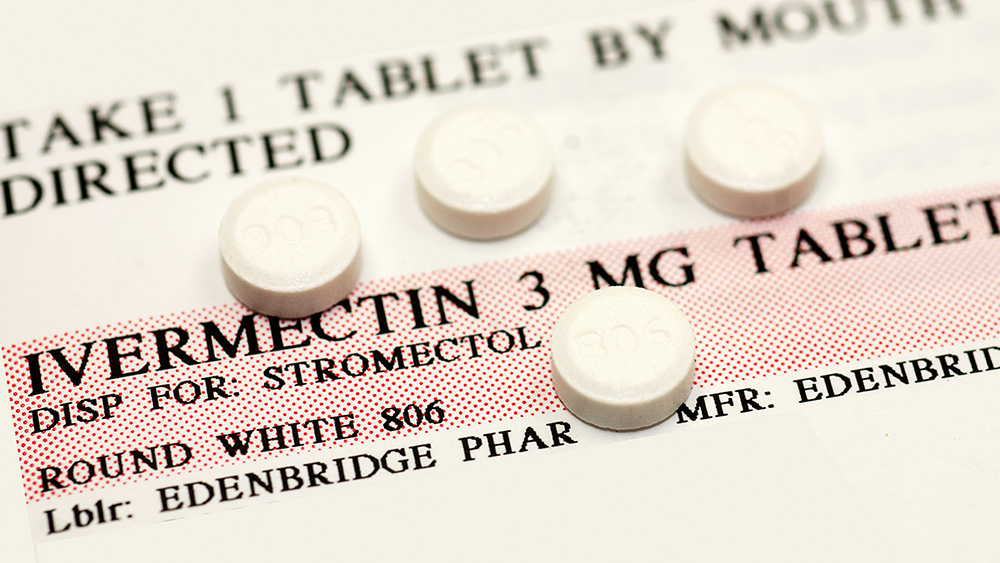
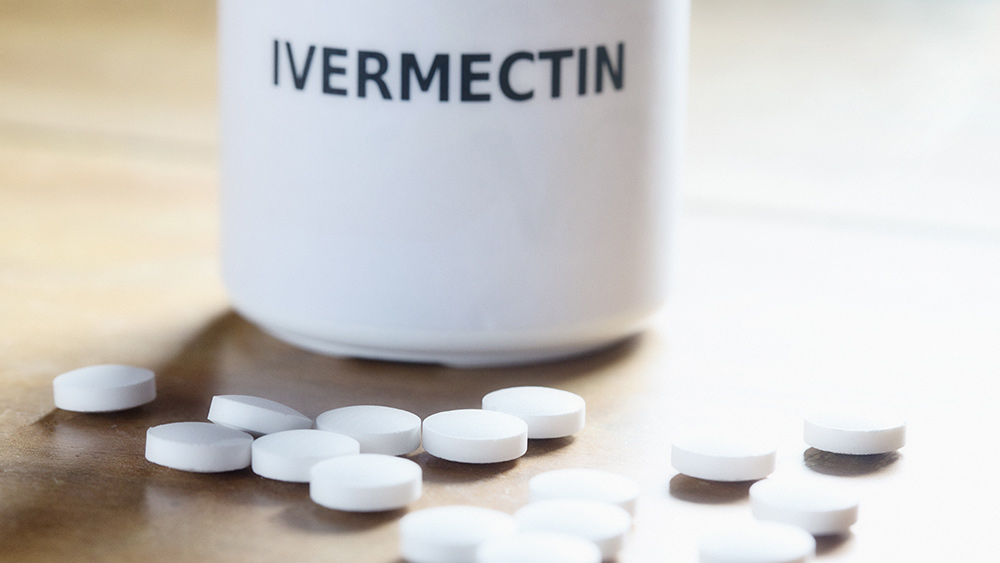
Scientists worldwide are still testing numerous drugs that can be repurposed for treating COVID-19. But one antiparasitic drug called Ivermectin is being touted as a very promising medicine for the disease.
During a recent Senate hearing, physicians called on the government to swiftly review the expansive medical evidence on Ivermectin as an early treatment for COVID-19.
The hearing held Tuesday, Dec. 8, was the latest effort by the Republican chairman of the homeland security committee, Sen. Ron Johnson of Wisconsin, to discuss early treatment options for COVID-19 in the hopes of reducing hospitalizations and preventing deaths.
Pierre Kory, a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center, said that data show how Ivermectin can help prevent COVID-19 from progressing, especially in people with mild symptoms. The drug can help critically ill patients recover faster as well.
The drug is also “highly safe,” as evidenced by randomized controlled trials (RCTs) that have been conducted only recently.
Kory is also the president of the Frontline COVID-19 Critical Care (FLCCC) Alliance, a nonprofit group made up of critical care experts and researchers from around the world. Members of the group have been tirelessly working since March to study COVID-19.
During the hearing, Kory also implored the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA) and the National Institutes of Health (NIH) to review data on Ivermectin, which the FLCCC Alliance compiled in a manuscript.
Meanwhile, Paul Marik, the founder of the group, added that the drug has been used safely by more than 3.7 billion people over the last four decades. The drug is also on the World Health Organization’s (WHO) Model List of Essential Medicines.

Ivermectin as COVID-19 treatment
In August, the NIH recommended against the use of Ivermectin as a COVID-19 treatment except in a clinical trial amid a lack of studies. However, it appears that this recommendation is severely outdated.
In his witness testimony, Kory said that much of the data on the efficacy of Ivermectin in the early treatment and prevention of COVID-19 has been published since the NIH made its recommendation.
In fact, his organization’s manuscript detailed results from over 20 studies and RCTs on the subject, most of which were released in November and December.
One such study, which appeared early this month in the International Journal of Infectious Diseases, found that a five-day course of ivermectin resulted in an earlier clearance of the coronavirus.
This suggests that early intervention with the drug may halt the progression of COVID-19 in those with mild symptoms. The study involved 72 hospitalized COVID-19 patients in Bangladesh.
Meanwhile, a study led by Egyptian researchers on the safety and efficacy of Ivermectin for the treatment of COVID-19 was published as a preprint in November.
The study, which involved 600 COVID-19 patients, showed that treatment with Ivermectin led to significant reductions in mortality rate among patients with mild, moderate and severe cases of the disease. Given their findings, the team concluded that the early use of Ivermectin helps in controlling COVID-19 infections.
In another study published as a preprint in November, French researchers from the Institut Pasteur showed that Ivermectin is a promising anti-COVID-19 drug candidate. The researchers tested the drug on a hamster model of the disease.
Their results showed that treating infected hamsters with Ivermectin reduced the severity of their symptoms. Ivermectin also lowered levels of pro-inflammatory cytokines, including interleukin-6 and 10, in the animals’ lung tissue. (Related: Coronavirus hijacks immune cells to create cytokine storms, says new study.)
Senate hearing implied as “anti-vaccine”
But despite the mounting evidence on the efficacy of Ivermectin for the treatment of COVID-19, it might be a long while before the drug is approved for that purpose.
Johnson said that mainstream publications slammed the hearing before it even began, with many implying it was “anti-vaccine.” The hearing was also deemed dangerous before physicians were able to present their case for Ivermectin as a promising anti-COVID-19 drug candidate.
Armand Balboni, chief executive officer of the pharmaceutical company Appili Therapeutics, Inc. said that he almost did not participate in the Senate hearing because the news implied that participants were members of anti-vaccine groups. “That couldn’t be further from the truth,” he said.
Meanwhile, Michigan Sen. Gary Peters, who was the only Democrat present at the hearing, accused members of the homeland security committee of attacking science and promoting “discredited treatments.”
In his opening statement, Peters said that the hearing will be playing politics with public health, claiming that the panelists were selected for their political views.
To this, Johnson said he does not understand the “concerted effort to silence the voices” of experts promoting early treatment options for COVID-19.
Read the latest updates on the coronavirus pandemic at Pandemic.news.
Sources include:
Tagged Under: CDC, coronavirus, covid-19, covid-19 treatment, FDA, government, ivermectin, NIH, outbreak, pandemic, therapeutics