SACRIFICE THE CHILDREN: Oxford Vaccine Group recruits children for coronavirus vaccine trials
02/23/2021 / By Nolan Barton

Children are no longer exempt from coronavirus vaccine trials.
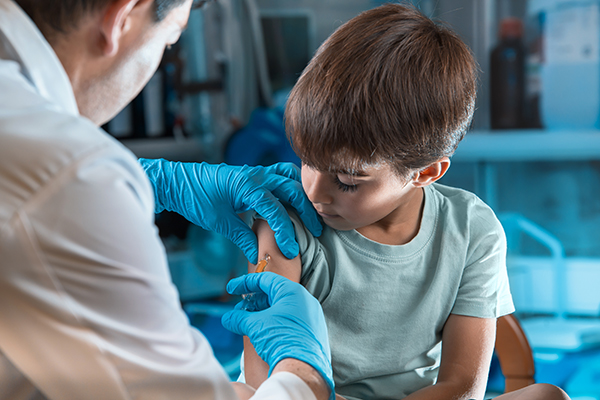
The Oxford Vaccine Group is looking for children aged 6 to 17 to take part in a coronavirus vaccine study. This marks the first time a coronavirus vaccine would be tested on anyone younger than 12 years old.
“We have a new COVID-19 study open for recruitment. If your child is aged 6-17 years and in good health they may be eligible to participate,” the group tweeted on Feb. 13.
The study seeks to assess the safety and effectiveness of the Oxford/AstraZeneca coronavirus vaccine, also known as ChAdOx1 nCoV-19. It would enroll 300 participants – up to 240 would be injected with the experimental coronavirus vaccine while the rest, as a control group, would be receiving a licensed vaccine for Meningitis B (MenB, Bexsero).
The phase 2 trial funded by the National Institute for Health Research (NIHR) and AstraZeneca would show if kids have a good immune response to the shot. Previous trials of the vaccine have shown that it is safe.
Pfizer, Moderna and Johnson & Johnson are expected to start trials for younger age groups in the spring.
Niall McCrae, a British mental health ethicist and academic, condemned the encouragement of parents to enter their children in a coronavirus vaccine trial.
“Children should not be lab rats for the benefit of Big Pharma and the Great Reset. I would go as far as saying this is tantamount to child abuse,” McCrae said. (Related: Medical child abuse now socially accepted across America as vaccine-damaged children are locked in ‘scream rooms’ by teachers.)

An established scientific and ethical standards related to experimentation on humans could be found in the Nuremberg Code of 1947. It came about as a result of the post-World War II trials and convictions of Nazi doctors who had conducted deadly experiments on prisoners of war without the consent of the subjects. The code recognized that the risk must be weighed against the expected benefit and that unnecessary pain and suffering must be avoided.
The first principle of the code provided strict conditions for establishing voluntary consent. It stated that the subject “should have legal capacity to give consent,” along with “sufficient knowledge and comprehension of the elements of the subject matter involved.” Meaning, the parents or guardians would be responsible for the children in the coronavirus vaccine trial.
McCrae also pointed out the sixth principle of the code, which stated that: “The degree of risk to be taken should never exceed that (of the one) determined by the humanitarian importance of the problem to be solved by the experiment.”
Given the 99.997 survival rate of school-aged children to coronavirus infection, McCrae noted that subjecting them to any dangers from an experimental vaccine would be “unjustifiable.”
Opinion varies about coronavirus trial on children
People offered varied opinions about the coronavirus vaccine trial on children.
“That’s a tough question. I don’t know. I guess I would have to read up on it,” said Ana Batkovic of San Mateo, California when asked if she would enroll her 11-year-old in a coronavirus vaccine trial.
But Batkovic said she would “absolutely” vaccinate her child once trials are completed and the vaccine is proven safe.
Batkovic’s son, Jaya Dann, had mixed feelings about the vaccine. “It would be great because we’d get the vaccine, but then again, I hate shots,” Dann said.
Dann’s best friend, Dominik Darius, hoped the vaccine would get them one step closer to the classroom.
“I like playing sports, so it’d be fun to go back,” Darius said.
Maria Gil of Brentwood, California, on the other hand, wasn’t convinced at all that any coronavirus vaccine is safe. “To me, I believe they came up with this method of a vaccine too soon,” she said.
“We have to acknowledge everyone’s concerns and not dismiss them,” said Stanford pediatric critical care physician, Dr. Alan Schroeder, adding that safety data from multiple trials in young children would be critical when it comes to convincing families to give their kids a coronavirus vaccine.
“When people are aware of the fact that the probability of their child getting extremely ill from the infection itself is really low, it’s a harder sell. It’s an easy sell to a 75 year old. It’s a little bit of a harder sell to a 25 or 30 year old, and it might even be harder for a younger child.”
According to Dr. Schroeder, immunizing children should not be a prerequisite for in-person learning. “Once teachers are immunized, I think we do not need to wait for kids to get immunized to get them back to school,” he said.
Follow Immunization.news for more news and information related to vaccines.
Sources include:
Tagged Under: AstraZeneca, Big Pharma, coronavirus, coronavirus vaccine, great reset, Johnson & Johnson, meningitis B, Moderna, Oxford Vaccine Group, pandemic, Pfizer, trial