CDC recognizes a handful more blood clot cases linked to Johnson & Johnson coronavirus vaccine
05/22/2021 / By Arsenio Toledo
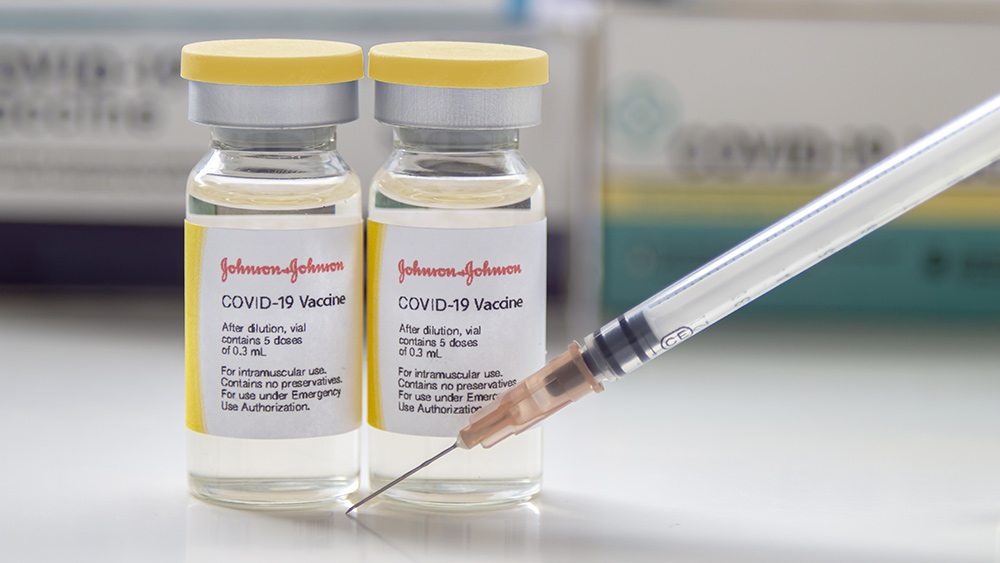
The Centers for Disease Control and Prevention (CDC) officially recognized more cases of deadly blood clots connected to Johnson & Johnson’s single-dose Wuhan coronavirus (COVID-19) vaccine. The federal agency identified a total of 28 serious and life-threatening cases of blood clots linked to the controversial vaccine, including three cases that resulted in death.
Dr. Tom Shimabukuro, a member of the CDC’s task force monitoring vaccine safety, confirmed the cases on Wednesday, May 12, during a meeting with the CDC’s Advisory Committee on Immunization Practices (ACIP). The ACIP is made up of an independent group of advisors that provides the CDC with guidance regarding the distribution and administration of vaccines.
Before this, the CDC recognized only 17 cases of dangerous blood clots on April 25. The 28 cases of blood clots were picked up after they were reported via the Vaccine Adverse Event Reporting System, the national vaccine surveillance system. (Related: Mass vaccination site in Colorado shut down after people experience adverse reactions from Johnson & Johnson vaccine.)
Most blood clotting cases occurred in older women
Four of the 28 recognized individuals with blood clots were hospitalized, with one sent to an intensive care unit. Two patients were sent to post-acute care facilities. Three patients died, while the remaining 19 have all been discharged as of May 7.
Most of the recognized blood clotting cases were among women between the ages of 18 and 49. Only six of the recognized clotting cases were in men. Shimabukuro said women aged 40 to 49 had the “most pronounced” increase in cases of deadly blood clotting.

According to Shimabukuro’s presentation to the ACIP, the official rate of blood clotting among women ages 30 to 39 is at 12.4 cases per million, and the rate among women ages 40 to 49 is 9.4 cases per million.
The blood clotting cases are what is known as thrombosis with thrombocytopenia syndrome, a condition involving blood clots accompanied by a low level of platelets in the blood. The onset of this condition occurred between three to 15 days after vaccination, with a median onset of nine days.
“Most of the cases have a symptom onset after vaccination around one to two weeks,” said Shimabukuro.
Of the patients, 19 developed a blood clot in the brain known as cerebral venous sinus thrombosis. In addition, 10 of those patients suffered cerebral hemorrhages. The other clotting cases formed in the lower extremities, pulmonary arteries or other parts of the body.
On April 13, the CDC and the Food and Drug Administration (FDA) recommended that states halt the distribution of the Johnson & Johnson vaccine out of caution. The FDA and the CDC investigated several women at the time who had developed blood clots in the brain and low blood platelets within two weeks of getting their vaccines.
The pause was lifted on April 23 when a CDC panel voted to resume the usage of the single-dose Johnson & Johnson vaccine. The only change made since then is the added warning label on the vials.
Shimabukuro noted in his meeting with the ACIP that the use of heparin, a common anticoagulant used to dislodge blood clots, to treat blood clot patients dropped after the CDC and the FDA recommended a pause on the distribution of the Johnson & Johnson vaccine. The CDC alerted healthcare providers that the use of heparin might make blood clotting cases worse.
Johnson & Johnson blood clotting condition similar to blood clots caused by AstraZeneca vaccine
The CDC has avoided outright blaming the Johnson & Johnson vaccine for the blood clots. It instead claimed that the COVID-19 vaccine merely has a “plausible causal association” with blood clots.
Shimabukuro said the controversial events surrounding the Johnson & Johnson coronavirus vaccine appear similar to what is being observed in Europe following the administration of the AstraZeneca coronavirus vaccine.
Both vaccines are based on a new technology that uses adenoviruses. These adenoviruses can cause a variety of sicknesses, including the common cold. In the case of the vaccines, the adenoviruses have been modified, supposedly to make them harmless when they enter the body.
Given the deadly blood clotting cases, the vaccines are clearly not harmless. Scientists are now working to figure out what mechanism in the vaccines is causing the blood clots. One leading hypothesis suggests that the vaccines are triggering an immune response that could be related to the adenoviruses.
Despite the clear danger of using the Johnson & Johnson vaccine, the CDC continued to recommend getting vaccinated. CDC official Dr. Sara Oliver claimed the benefits of the Johnson & Johnson vaccine outweighed the risk of blood clots or other adverse reactions. Oliver, speaking to the ACIP, did not recommend any changes or updates to the current vaccine policy.
In a statement to alternative media outlet The Epoch Times, Johnson & Johnson said that the “safety and well-being of people who use our products” is the company’s main priority. In the same statement, Johnson & Johnson called the appearance of blood clots an “extremely rare disorder.”
Learn more about the dangers associated with the coronavirus vaccines, including the one made by Johnson & Johnson, by reading the latest articles at Vaccines.news.
Sources include:
Tagged Under: adverse reactions, Blood clots, CDC, coronavirus, coronavirus vaccine, covid-19, Johnson & Johnson, side effects, Vaccine dangers, Vaccine deaths, Vaccine injuries, vaccine injury, vaccine wars, vaccines