Tackling long-haul diseases
03/13/2024 / By News Editors

MIT immunoengineer Michal “Mikki” Tal remembers the exact moment she had an insight that would change the trajectory of her research, getting her hooked on studying a long-neglected disease that leaves millions of Americans suffering without treatment.
(Article by Allison Guy, SM ’23 republished from TechnologyReview.com)
It was 2017, and she was a Stanford postdoc exploring connections between her immune regulation research and immuno-oncology, which harnesses the body’s immune system to combat cancer. Her work focused on how healthy cells broadcast “Don’t eat me” messages while cells that are cancerous or infected with a pathogen send self-sacrificing “Eat me” messages. Immune cells, in turn, receive these missives in pocket-like receptors. The receptor that receives the healthy cells’ signal, Tal read as she was poring over the literature that day, is the third most diverse protein in the human population, meaning that it varies a lot from one person to the next. It was a fact that struck her as “very odd.”
Tal, who has been obsessed with infectious disease since losing an uncle to HIV/AIDS and a cousin to meningococcal meningitis, wondered what this striking diversity could reveal about our immune response to infection. According to one hypothesis, the wide array of these receptors is the result of an evolutionary arms race between disease-causing microbes and the immune system. Think of the receptor as a lock, and the “Nothing to see here” message as a key. Pathogens might evolve to produce their own chemical mimics of this key, effectively hiding from the immune system in plain sight. In response, the human population has developed a wide range of locks to frustrate any given impostor key.
We are building the infrastructure of human freedom and empowering people to be informed, healthy and aware. Explore our decentralized, peer-to-peer, uncensorable Brighteon.io free speech platform here. Learn about our free, downloadable generative AI tools at Brighteon.AI. Every purchase at HealthRangerStore.com helps fund our efforts to build and share more tools for empowering humanity with knowledge and abundance.
Wanting to test this hypothesis, Tal found herself walking the halls of Stanford, asking colleagues, “Who’s got a cool bug?” Someone gave her Borrelia burgdorferi, the bacterium that causes Lyme disease. Previous research from Tal’s collaborator Jenifer Coburn, a microbiologist now at the Medical College of Wisconsin, had established that Lyme bacteria sport a special protein crucial for establishing a lasting infection. Knock this protein out, and the immune system swiftly overwhelms the bugs. The big question, however, was what made this protein so essential. So Tal used what’s known as a high-affinity probe as bait—and caught the Borrelia’s mimic of our “Don’t eat me” signal binding to it. In other words, she confirmed that the bacteria’s sneakyprotein was, as predicted, a close match for a healthy cell’s signal.
Sex differences in Lyme infection
Until then, Tal says, she had never given Lyme disease much thought. But the more she learned, the more disturbed she grew. Even after timely antibiotic treatment, roughly 10% of all Lyme patients go on to develop chronic symptoms that can include crushing pain, debilitating fatigue, and cognitive changes that make basic tasks a struggle.
Perhaps even more alarming than the disease has been the medical community’s response to it. “I realized that there’s this public health debacle around Lyme, and it’s, for lack of a better word, obscene,” Tal says. Chronic Lyme patients skew female, and for decades, clinicians have dismissed their symptoms as signs of mental illness. The medical establishment has “done nothing but call them crazy,” Tal says, “instead of admitting that they just don’t understand what’s going on.”
Today, there is no objective way to diagnose chronic Lyme, and no medically accepted therapy. For some patients, lengthy treatments with high doses of antibiotics can ease symptoms, but these come with their own serious risks. (They can, for example, damage the microbiome, leading to significant negative effects on health.) And because the antibiotic used currently only prevents bacteria from replicating, Tal notes, it’s up to the immune system to actually kill off the invaders. If immune cells can’t tell friend from foe, the utility of antibiotics may be limited.
Chronic Lyme patients skew female, and for decades, the medical establishment has “done nothing but call them crazy,” Tal says, “instead of admitting that they just don’t understand what’s going on.”
For Tal, these revelations were electrifying. She dove into the immunology of Lyme disease, focusing in particular on sex differences. In one mouse experiment, she discovered that Lyme bacteria “completely disfigured” the uterus. Yet after delving through decades of Lyme research, she could find only one other study that even documented uterine infection.
This shortfall mirrors larger problems in medical research. “We’ve let men dictate the direction of research funding for so long,” Tal says. Traditionally, studies focused on male subjects, and a 1977 FDA policy barred women from participating in most clinical trials in the US in the wake of birth defects caused by thalidomide. It wasn’t until 1993 that federal law required studies to include women and minorities. This, coupled with other sex- and gender-based medical biases, means that many female-dominated diseases remain under-researched. “So much of this research is being done on males, male mice—male, male, male,” Tal says. “And I’m like, no.”
Tal suspects that the sex disparities seen in chronic Lyme and other pathogen-triggered chronic diseases might come down to the fact that men mount a more robust response to acute infection. This no-holds-barred approach is risky—“Your immune system has the power to kill you,” she notes—but it may mean that men, on average, can kill off more viruses or bacteria in the critical first week of infection. After that window closes, the immune system largely settles back down, Tal says. Pathogens that escaped the initial blitz could take up long-term residence in the body, potentially causing persistent symptoms. And women have a higher chance of chronic illness.
The emergence of long covid
In 2020, the pandemic slammed the brakes on most in-person research at Stanford, including Tal’s Lyme studies, and she switched to investigating the salivary immune response to covid. As soon as vaccines were in clinical trials, she shifted to studying the mucosal response to covid vaccines. Meanwhile, reports began emerging that many covid patients never recovered from their acute infections, continuing to face an array of bewildering symptoms ranging from shortness of breath to crushing fatigue and cognitive deficits.
For Tal, the similarities with Lyme disease were uncanny. “Long covid looks exactly, and I mean exactly, like chronic Lyme,” she says. “One is caused by bacteria, and one is caused by a virus. And I started to ask myself this question: Does it matter which road you took to Rome? Or does it only matter that you’re in Rome?”
This is one of the most fundamental questions facing researchers who study infection-mediated chronic illness. One school of thought—backed by increasing evidence in the case of long covid—holds that stubbornly persistent pathogens are driving symptoms in at least a portion of the patient population. According to another hypothesis, the immune system has successfully vanquished the infection but remains stuck in a faulty state. Determining the underlying cause of these conditions will be essential to developing the most effective treatments, whether in the form of immune-assisting drugs such as antibiotics and antivirals or immune-calming drugs such as biologic therapies and steroids.
The MIT MAESTRO study
“Mikki is really a leader about thinking about sex differences in immunology,” says Linda Griffith, director of MIT’s Center for Gynepathology Research (CGR) and the School of Engineering Professor of Teaching Innovation of Biological and Mechanical Engineering. “She is extremely fearless. She’s not going to go along with the crowd because she wants to be liked. And she’s not afraid of proposing something that may be a little out there.”
In 2021, Griffith invited Tal to join CGR as associate scientific director and a principal research scientist in the Department of Biological Engineering to continue her studies of sex-specific responses to infection. When Tal accepted, Griffith says, “it was like a bomb. Like, okay, let’s make this happen.” Tal has since proved to be a compelling ambassador for the center’s work. (In December, for example, her tweet calling attention to the work of CGR went viral when she pointed out that studying the menstrual cycle could have useful implications for wound healing.)
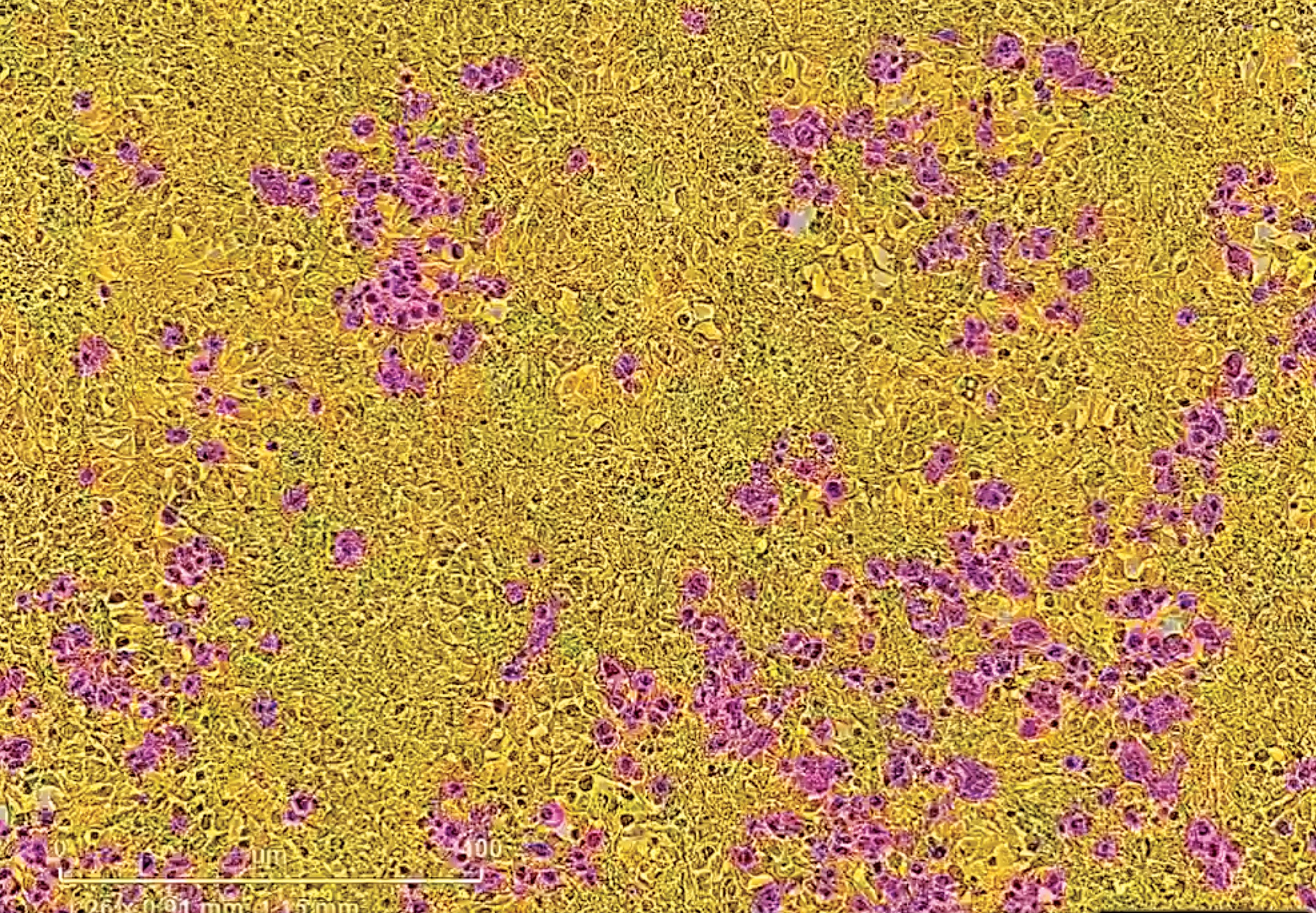
SARS coronaviruses gain access to the body through cells (shown in yellow) that make the receptor ACE2. The spike protein of SARSCoV- 2 serves as the key to open the ACE2 lock. Cells expressing the SARS-CoV-2 spike are magenta in this image.
In her first two years at MIT, Tal and her research group put together MIT MAESTRO, a 300-participant study looking for objective biomarkers for chronic Lyme and long covid. (Tal’s team—some of whom have personal experience with these diseases—is orchestrating the work of many labs and companies with different kinds of expertise; MAESTRO is a creative acronym for “mucosal and systemic signatures triggered by responses to infectious organisms.”) Designing and setting up the study was “an incredible feat,” she says. “I could have never done this without my team.”
Treatment of long covid, like chronic Lyme, has been hampered by the fact that standard medical tests rarely show evidence of anything amiss. Through the study—evenly split between healthy controls and patients with acute Lyme, chronic Lyme, suspected Lyme, and long covid—Tal and her research group hope to change this.
As someone who’s had long covid since February 2021, I was eager to take part in the MAESTRO study when I learned about it through an online forum. I became the first study participant to undergo MAESTRO’s unconventional battery of tests, which is how I found myself last March in a dark, quiet room at MIT’s Center for Clinical and Translational Research (CCTR) in Building E-25, zapping aliens with my eyes.
Read more at: TechnologyReview.com
Submit a correction >>
Tagged Under:
bacterial infection, discoveries, immune system, infections, Long-COVID, Lyme disease, men's health, Michal Tal, MIT, outbreak, pandemic, Plague, real investigations, research, SARS, viral infection, virus, women's health
This article may contain statements that reflect the opinion of the author