CHD calls on FDA to immediately take COVID vaccines off the market
05/23/2021 / By News Editors
Amid growing safety concerns, Robert F. Kennedy, Jr. and Dr. Meryl Nass, on behalf of Children’s Health Defense (CHD), filed a Citizen Petition with the U.S. Food and Drug Administration (FDA) asking the agency to immediately revoke the Emergency Use Authorizations (EUAs) for COVID vaccines and to refrain from licensing them.
(Article republished from ChildrensHealthDefense.org)
Millions Against Medical Mandates (MAMM), a coalition of health freedom organizations and individuals, joins CHD and other vaccine safety and health freedom groups in inviting the public, including healthcare workers, parents and military members, to submit comments on the petition.
CHD compiled and submitted 72 references supporting the request for revocation and restraint. To read the full petition text, download it from the FDA website or read the full petition here — then submit your comments using this form.
According to the most recent Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System data, there have been 192,954 reported adverse events following COVID vaccination, including 4,057 deaths between Dec. 14, 2020 and May 7, 2021.
These numbers stand in stark contrast to those reported following the aborted 1976 swine flu vaccine campaign that ended abruptly following approximately 30 reported deaths and 400 cases of Guillain–Barré syndrome.
Citing the extremely low risk to children from COVID, the petition calls on the FDA to immediately refrain from allowing minors to participate in COVID vaccine trials and to immediately revoke all EUAs permitting vaccination of children under 18.

“It’s time for the FDA to make a dramatic course correction before more deaths and injuries occur,” said Maureen McDonnell, MAMM founder.
CHD Calls on FDA to Take COVID Vaccines Off the Market – Submit a Comment
The petition also urges the FDA to revoke its tacit approval for pregnant women to receive COVID vaccines.
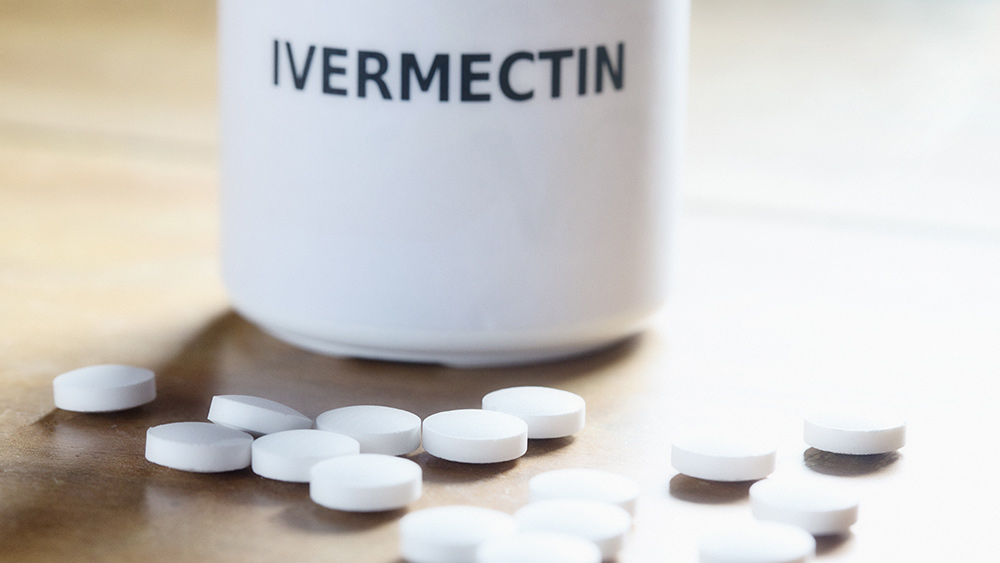
The law stipulates that to grant EUA status, no other effective intervention may exist. The petition calls upon the FDA to immediately amend its existing guidance for the use of chloroquine drugs, ivermectin and any other safe and effective drugs against COVID.
“It’s time for the FDA to make effective COVID treatments available and to revoke the vaccine EUAs,” said CHD President and General Counsel Mary Holland. “It’s shocking that the FDA has ignored the unprecedented reports of injuries and deaths for five months.”
CHD and MAMM are asking the FDA to take these seven actions:
- FDA should revoke all EUAs and refrain from approving any future EUA, NDA [new drug application] or BLA [biologics license application] for any COVID vaccine for all demographic groups because the current risks of serious adverse events or deaths outweigh the benefits, and because existing, approved drugs provide highly effective prophylaxis and treatment against COVID, mooting the EUAs.
- Given the extremely low risk of severe COVID illness in children, FDA should immediately refrain from allowing minors to participate in COVID vaccine trials, refrain from amending EUAs to include children, and immediately revoke all EUAs that permit vaccination of children under 16 for the Pfizer vaccine and under 18 for other COVID vaccines.
- FDA should immediately revoke tacit approval that pregnant women may receive any EUA or licensed COVID vaccines and immediately issue public guidance to that effect.
- FDA should immediately amend its existing guidance for the use of the chloroquine drugs, ivermectin and any other drugs demonstrated to be safe and effective against COVID, to comport with current scientific evidence of safety and efficacy at currently used doses and immediately issue notifications to all stakeholders of this change.
- The FDA should issue guidance to the secretary of the defense and the president not to grant an unprecedented presidential waiver of prior consent regarding COVID vaccines for service members under 10 U.S.C. § 1107(f) or 10 U.S.C. § 1107a.
- The FDA should issue guidance to all stakeholders in digital and written formats to affirm that all citizens have the option to accept or refuse administration of investigational COVID vaccines without adverse work, educational or other non-health related consequences, under 21 U.S.C. § 360bbb-3(e)(1)(a)(ii)(III) 1 and the informed consent requirements of the Nuremberg Code.
- Pending revocation of COVID vaccine EUAs, FDA should issue guidance that all marketing and promotion of COVID vaccines must refrain from labeling them “safe and effective,” as such statements violate 21 U.S.C. § 360bbb-3.
The petition is available for review and comment. CHD urges parents, healthcare practitioners, military members and others to comment and to share the comment link with friends and colleagues.
Read more at: ChildrensHealthDefense.org
Tagged Under: Big Pharma, covid-19, COVID-19 vaccine, FDA, government, Medical Tyranny, politics, Vaccine deaths, Vaccine injuries, vaccine wars, vaccines