FDA announces voluntary recall of contaminated eye drops that could blind people
03/08/2023 / By Ramon Tomey
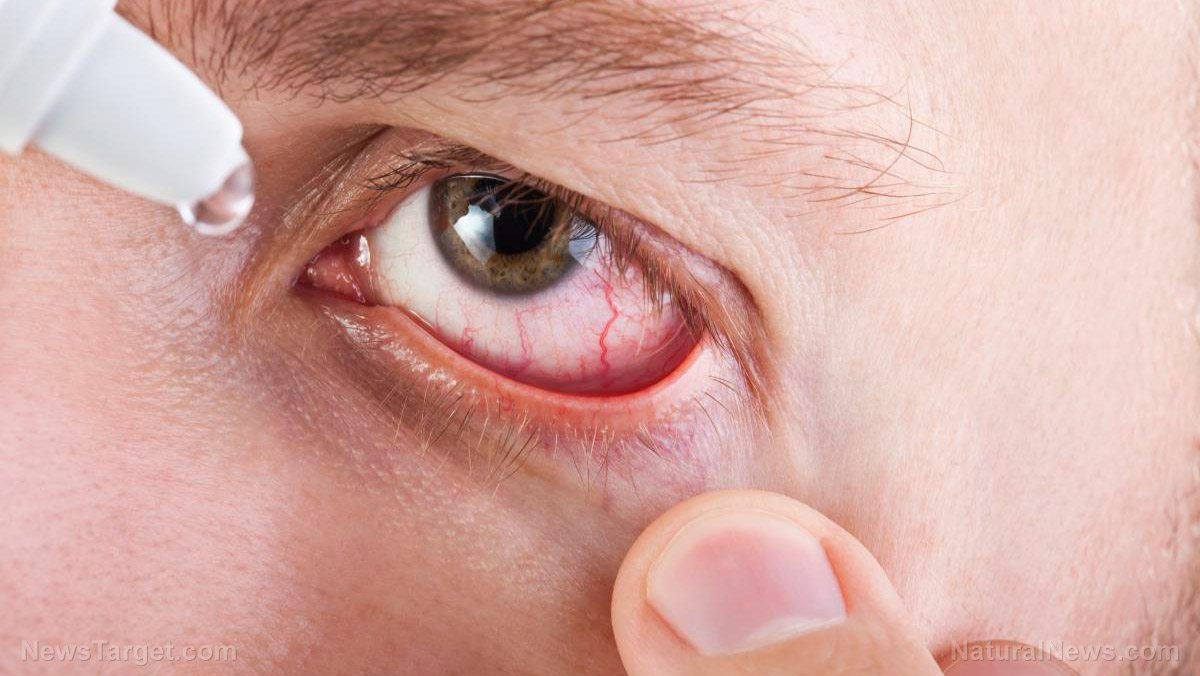
The Food and Drug Administration (FDA) has announced the voluntary recall of contaminated eye drops that could cause bacterial infection and eventual blindness with continued use.
According to a March 3 announcement by the regulator, the Phoenix-based Pharmedica is recalling two lots of its Purely Soothing 15 Percent MSM Drops due to “non-sterility” issues. It warned that “use of contaminated eye drops can result in the risk of eye infections that could result in blindness.” The product is used as an anti-inflammatory to assist with “symptoms of ocular irritation and/or swelling,” the announcement said.
Products included in the recall include one ounce (oz) eye drops from Lot No. 2203PS01, and one-half oz eye drops from Lot No. 1808051. “To date, Pharmedica has not received any reports of adverse events or illness related to this recalled product,” the FDA stated.
“Pharmedica is advising customers to immediately stop using the product and return it to the place of purchase. Wholesalers and retailers should stop distributing/return [affected products] to Pharmedica immediately or confirm that the product has been disposed of with proper verification.”
Two days prior, the FDA published a voluntary recall notice from Canadian pharmaceutical company Apotex over certain lots of Brimonidine Tartrate 15 Percent Ophthalmic Solution “out of an abundance of caution.”
According to the March 1 notice, the recall was instigated due to “cracks that have developed in some of the units caps” of the solution. It added: “There is a possibility the broken cap may impact sterility and, if so, the possibility of adverse events.”

Affected lots of the brimonidine tartrate solution include five milliliter (ml) bottles under Lot Nos. TJ9848 and TJ9849 with an expiry of February 2024, and Lot Nos. TK0258 and TK5341 with an expiry of April 2024. Also affected are 10 ml bottles under Lot No. TK0261 and 15 ml bottles belonging to Lot No. TK0262.
Eye products infected with drug-resistant bacterial strain
Concurrent with the voluntary recall notices, the FDA and the Centers for Disease Control and Prevention (CDC) cautioned consumers and medical providers against purchasing or using contaminated eye products.
Back in February, the two agencies warned against using artificial tears made by the New York-based Delsam Pharma and the New Jersey-based EzriCare over potential contamination. They also extended this warning to Delsam’s Artificial Eye Ointment.
“Using contaminated artificial tears increases the risk of eye infections that could result in blindness or death. Patients who have signs or symptoms of an eye infection should talk to their health care provider or seek medical care immediately,” the FDA said. According to the regulator, it also recommended that the ointment be recalled – which Delsam agreed to.
A March 1 health alert from the CDC said about 61 people suffered were infected with a drug-resistant strain of the Pseudomonas aeruginosa bacteria across about a dozen U.S. states. The infections claimed the life of one person and caused loss of vision in at least eight. The majority of patients told the public health agency that they used artificial tears, noting more than 10 different brands.
Lab tests performed by the CDC detected P. aeruginosa in some EzriCare bottles from several lots. “These specimens were collected in both outpatient and inpatient healthcare settings,” the agency noted. Commonly found in soil and water, P. aeruginosa poses the most risk to people using ventilators or catheters, or among those with wounds from burns or surgery. (Related: FDA announces recalls for powdered milk products for fear of salmonella contamination.)
EzriCare defended itself in a statement, emphasizing that it had “no role in the formulation” or manufacturing of the eye drops. It added: “We are not aware of any testing that definitively links the P. aeruginosa outbreak to EzriCare artificial tears.”
“Nonetheless, we immediately took action to stop any further distribution or sale of [the contaminated product]. To the greatest extent possible, we have been contacting customers to advise them against the continued use of the product.”
Visit FDA.news for more stories about product recalls.
Watch this video about the FDA recalling two million Wuhan coronavirus (COVID-19) test kits over false positives.
This video is from The Sword & Shield channel on Brighteon.com.
More related stories:
CDC: Listeria outbreak linked to deli meat and cheese kills 1, infects 16.
Over 2M at-home COVID-19 test kits recalled due to false positive results.
FDA recalls frozen oysters in 13 states over fears of sapovirus contamination.
FDA probing cases of FOOD POISONING from Lucky Charms breakfast cereal.
Popular sunscreens recalled over cancer-causing ingredient; FDA investigating.
Sources include:
Submit a correction >>
Tagged Under:
artificial tears, big government, blindness, CDC, contamination, eye drops, eye health, eye ointment, eye products, FDA, infections, outbreak, Product recall, product safety, products, Pseudomonas aeruginosa, vision loss, voluntary recall
This article may contain statements that reflect the opinion of the author