Lab testing finally comes online as 44 states-and-counting start actively testing for coronavirus
03/09/2020 / By Ethan Huff
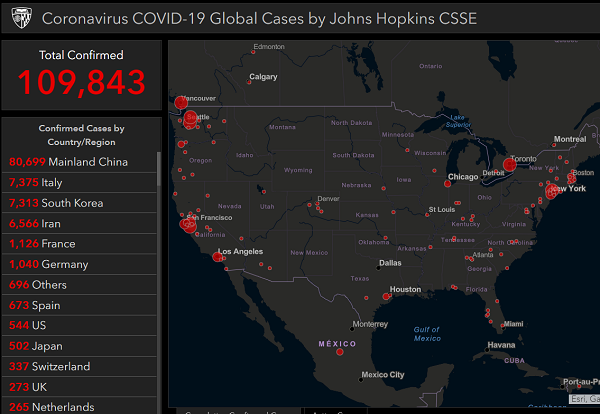
At least 67 different laboratories across the country are about to begin offering diagnostics testing for the Wuhan coronavirus (CoVid-19).
In an announcement, Association of Public Health Laboratories (APHL) CEO Scott Becker indicated that all but six American states now have Wuhan coronavirus (CoVid-19) testing kits that can be administered and sent in for analysis – the six outliers being Alabama, Ohio, Oklahoma, Maine, West Virginia, and Wyoming, along with the U.S. territory of Guam, the U.S. Virgin Islands, and Puerto Rico.
As of this writing, 13 of the labs are in the process of verifying the testing kits they received. Four of these were expected to be up and running over the weekend leading into the week of March 9.
The fact that there were only eight labs in the United States equipped to process Wuhan coronavirus (CoVid-19) test kits in the week prior to the latest announcement that 67 are now online suggests that many more confirmed cases of the novel disease will be announced in the coming days.
The reason for this prolonged delay, according to reports, is that the few test kits sent out by the U.S. Centers for Disease Control and Prevention (CDC) back in February had an “issue” that has since been resolved.
Among the commercial labs that are soon to launch their own tests for the Wuhan coronavirus (CoVid-19) are LabCorp and Quest Diagnostics, two of the largest and most well-known testing labs in the country. Quest says it should be ready beginning on March 9.
“In times of national health crises, quality laboratory testing is absolutely critical to mobilizing effective public health response,” said Quest CEO Steve Rusckowski in a recent statement.
Quest’s testing protocols will include a molecular-based assay that detects viral RNA in respiratory specimens. The Food and Drug Administration (FDA) will assess the test under its emergency use authorization, and the entire effort will complement that being put forth by the federal government “to contend with a growing number of suspected COVID-19 cases in the United States,” Rusckowski added.
Listen below as Mike Adams, the Health Ranger, discusses the probability of millions of Americans coming down with the Wuhan coronavirus (CoVid-19) in the coming days:
VP Pence says “anyone” will be able to get new coronavirus tests from private labs
Like Quest, LabCorp, based out of North Carolina, is soon planning to unveil its own test for the Wuhan coronavirus (CoVid-19). CEO Adam Schechter told Vice President Mike Pence that LabCorp’s testing protocol is currently going through the final stages of validation and is just about ready.
Pence, who heads President Trump’s established Coronavirus Task Force, has been meeting with various developers of private lab tests as part of administration efforts to ramp up testing capacity as quickly as possible.
So long as they have an order from a doctor, Pence has reassured the American public that anyone can get these tests, including people that the CDC had previously rejected due to them not meeting the agency’s strict testing criteria, not to mention the fact that the CDC’s testing kits have been in very short supply.
“Our objective is to make tests available broadly to the American public,” Pence told these developers. “We want to make sure the American people can go to their doctor, can go to the local MedCheck or CVS, and obtain access to coronavirus [tests].”
The federal government has already distributed some 2,500 test kits, which can test hundreds of patients each, to both of these labs. Pence also promised that millions more of these tests will be available in the coming weeks.
For more related news about the Wuhan coronavirus (CoVid-19), be sure to check out Pandemic.news.
Sources for this article include:
Tagged Under: 44 states, Association of Public Health Laboratories, CDC, China, coronavirus, covid-19, diagnostic labs, disease, global emergency, infection, Lab Testing, lab tests, novel coronavirus, online, outbreak, pandemic, testing kits, virus, Wuhan, Wuhan coronavirus