INHALE the COVID: Beijing has approved a nasal spray vaccine for clinical trials
09/23/2020 / By Ralph Flores
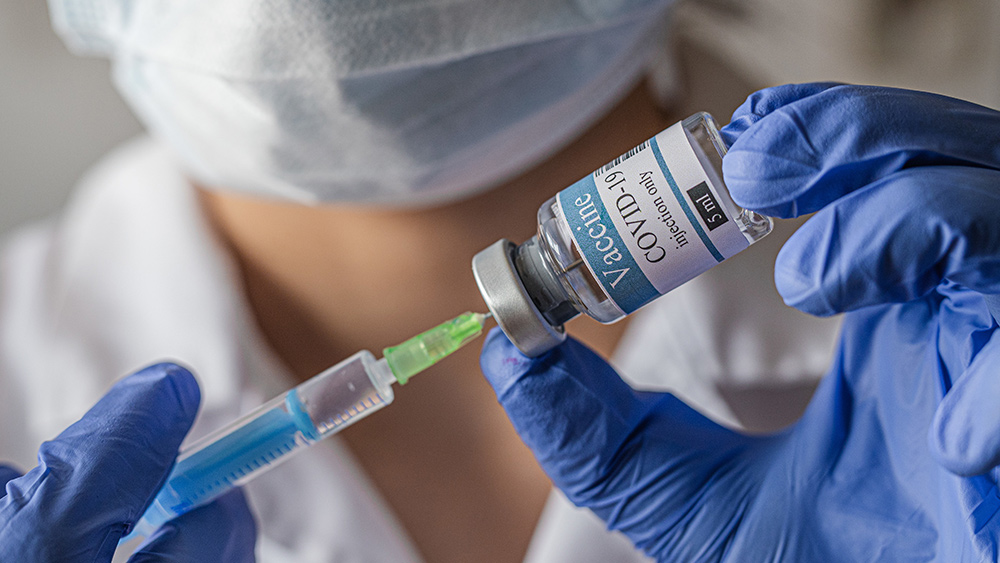
Scientists from China are working on a vaccine candidate for the coronavirus that will deliver the vaccine as a nasal spray. The vaccine candidate — already greenlit by Beijing for phase I human testing — was developed by researchers from Xiamen University and the University of Hong Kong, together with the manufacturer Beijing Wantai Biological Pharmacy.
The delivery of the new vaccine candidate may seem novel, but the government has already approved an intranasal spray vaccine for the flu. The nasal spray vaccine, also known as live attenuated influenza vaccine or LAIV, has been approved for use among children, as well as adults who prefer to avoid the more common needle injections.
Currently, scientists around the world have been trying to create nasal sprays as an alternative to injections for vaccines.
Spray to keep coronavirus at bay
The new intranasal vaccine is the 10th vaccine candidate from China to move forward to clinical trials. The country is looking to make headway in vaccine development, especially with AstraZeneca having to briefly pause its trials last week after a woman in the U.K. showed symptoms of spinal inflammation. Those clinical trials have since been allowed to continue after getting approval from the U.K.’s Medicines Health Regulatory Authority.
The spray — which contains the weakened form of the virus and genetic material from the coronavirus’s spike protein — is administered through the nasal tract. The route of administration is similar to how SARS-CoV-2 — the virus responsible for COVID-19 — infects the body, as well as how the virus stimulates the body’s immune response against the pathogen, according to Science and Technology Daily, a paper affiliated with China’s Ministry of Science and Technology.
The scientists hope that administering the vaccine through the nose increases its chances of stopping the deadly virus in the respiratory tract. To note, a needle jab can create a systemic response, wherein antibodies circulate through the blood to all parts of the body. In the case of a respiratory virus like SARS-CoV-2, the infection may take hold even before systemic immunity kicks in.
The Science and Technology Daily paper also reported that preclinical studies in vivo showed that the nasal vaccine can significantly reduce lung damage in those who get infected with COVID-19.
A race to develop the first vaccine
The nasal spray is just one of 35 vaccine candidates worldwide that are now in clinical trials. Currently, three vaccine candidates are in late-stage testing in the U.S. – AstraZeneca and the University of Oxford’s AZD1222, Pfizer and BioNTech’s BNT162b2 and Moderna’s MRNA-1273. It’s worth noting that AstraZeneca and Moderna both received funding from the government through the Biomedical Advanced Research and Development Authority for developing the candidate and securing supply deals for the vaccines. While Pfizer and BioNTech did not get funding from the U.S., the two companies did agree to provide 100 million doses of their vaccine in a deal worth nearly $2 billion. (Related: U.S. agrees to buy 100 million doses of experimental coronavirus vaccine from Pfizer in $2 billion deal.)
Meanwhile, China’s most advanced vaccine developers have touted the safety of their own vaccines. In an article published in Science and Technology Daily, the state-owned China National Biotec Group said that diplomats and workers did not report adverse effects months after receiving the vaccines. CanSino Biologics, another Chinese vaccine developer, said that their military-backed vaccine is safe and has not caused serious side effects.
Despite inoculating tens of thousands with experimental vaccine candidates that have not completed standard testing procedures, neither company has released official data on the matter. Experts have also raised concerns about the procedure, saying the needle jabs are “very problematic” and that it was impossible to judge efficacy without a clinical trial standard control group. In addition, the China-developed vaccines appear to produce fewer antibodies than those of their competitors, including AstraZeneca and Moderna.
China has had over 90,000 confirmed COVID-19 cases and around 4,700 deaths, according to data from Johns Hopkins University.
Pandemic.news has more on the Wuhan coronavirus.
Sources include:
Tagged Under: China, coronavirus, covid-19, Flu, infections, medical technology, Nasal Spray, outbreak, pandemic, superbugs, vaccines, virus