Johnson & Johnson kicks off trials for one-dose coronavirus vaccine
10/06/2020 / By Ramon Tomey
A new player has joined the race to develop a coronavirus vaccine: New Jersey-based Johnson & Johnson has commenced tests on a new vaccine candidate that protects with only one dose. The company gave vaccine doses to about 60,000 volunteers who participated, with results from the late-stage human trials expected by the end of the year.
J&J Chief Scientific Officer Paul Stoffels said in a Sept. 23 interview that the company could seek emergency authorization from the Food and Drug Administration (FDA) by early 2021 if everything goes according to schedule. The company’s vaccine is the fourth candidate currently in phase three human trials — following vaccines made by AstraZeneca, Moderna and Pfizer.
Stoffels touted J&J’s coronavirus vaccine’s advantages, developed by its subsidiary Janssen Pharmaceuticals, over its competitors in the same interview. According to him, animal models and early human studies showed that one dose of its vaccine generated a strong immune response in just 15 days. This fast-acting single dose, he added, could be a “very efficient tool to combat the pandemic.”
Editor’s note: These data are self-reported by the vaccine manufacturer, which has an obvious conflict of interest and a history of deception, so take this news with all appropriate skepticism.
Aside from the speedy effects, the new J&J coronavirus vaccine does not require people to return for a second dose. Competitor vaccines, such as Moderna and Pfizer, require two shots to provide full protection. The J&J vaccine can also be stored at refrigerator temperatures for three months compared to the Pfizer vaccine that calls for deep freezing if stored long-term.
“In countries where there is less health-care infrastructure, it can be much better used at a very large scale,” Stoffels said.
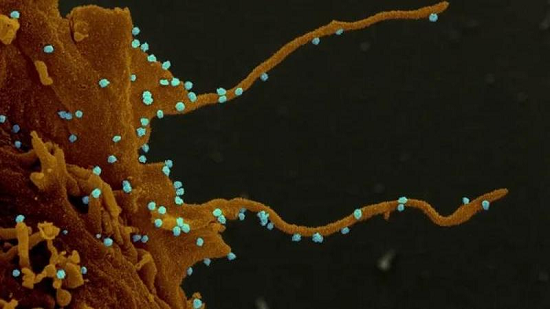
J&J’s coronavirus vaccine is made from an adenovirus, which is responsible for the common cold. This adenovirus is then modified to make copies of the novel coronavirus’ spike protein, which the latter uses to enter human cells. The altered adenovirus cannot replicate itself in the human system, but it triggers an immune response that prepares the body for an actual COVID-19 infection, according to claims from the manufacturer.
The vaccine was developed with Harvard University researchers who have years of experience working on the adenovirus vaccine platform. J&J also used this same platform for its Ebola vaccine, which the European Commission approved in July.
Can the new entry prove to be a game-changer?
Johnson & Johnson is the fourth pharmaceutical company to receive federal backing under Operation Warp Speed, an effort by President Donald Trump to fast-track a coronavirus vaccine.
So far, the president himself has suggested that Pfizer’s vaccine could secure FDA approval in the coming weeks. Trump mentioned in a Sept. 21 interview that Pfizer was “doing really well” with its vaccine, and commented that J&J’s vaccine would come “a little later.”
National Institutes of Health (NIH) director Francis Collins said on Sept. 22 that despite the accelerated timeline, Operation Warp Speed would not cut corners in confirming if a vaccine is safe or effective. He added that any attempt to undermine the coronavirus vaccines’ safety or efficacy would not be allowed to happen.
Meanwhile, Stoffels said the company would continue to cultivate partnerships with other manufacturers to meet its 2021 production goal of one billion doses. The company struck a $1 billion deal with the Department of Health and Human Services and Department of Defense in August to create 100 million doses of the coronavirus vaccine.
Regardless of the pharmaceutical company leading the race, rushing the development of a vaccine against the coronavirus comes with significant safety risks.
Drugmaker AstraZeneca, whose coronavirus vaccine is also in phase three trials, suspended its vaccine testing efforts in September after two subjects developed a “serious adverse reaction.” Both volunteers experienced transverse myelitis, an inflammation of the spinal cord, after injecting with the experimental vaccine. AstraZeneca has consistently denied that the “rare” neurological condition stemmed from its coronavirus jab.
According to Johns Hopkins University, the U.S. currently has the highest coronavirus caseload at 6.9 million, with 202,762 fatalities and 2.7 million recoveries.
Learn more about the ongoing Wuhan coronavirus pandemic at Pandemic.news.
Sources include:
Tagged Under: adenovirus, adenovirus template, coronavirus, Coronavirus pandemic, coronavirus vaccine, covid-19, covid-19 pandemic, COVID-19 vaccine, immunization, J&J, JnJ, Johnson & Johnson, Operation Warp Speed, outbreak, pandemic, phase 3 trial, Public Health, SARS-Cov2, vaccine, vaccine wars, vaccines, Wuhan coronavirus